The Gains and Pains of Using AI in the Pharmaceutical Industry
HIT Consultant
OCTOBER 25, 2022
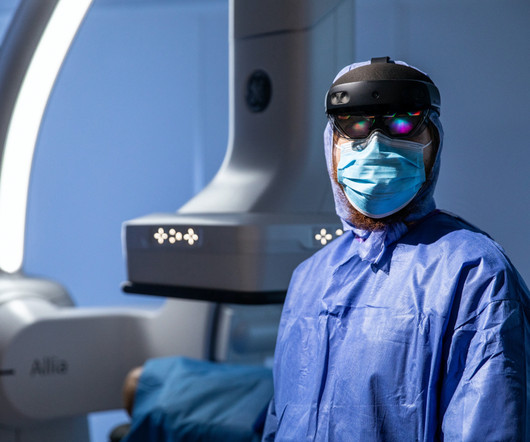
Contributing factors to this included larger trial sizes, the need to assess health technology, the requirement to provide data on comparative drugs’ effectiveness, and most importantly the high failure rate. A study in 2020 concluded that the estimated median capitalized research and development cost per product was $985 million.












Let's personalize your content